"The 3D visualization of subcellular structures only seen in 2D views was very satisfying," says Cahoon. "Some were just as you would imagine but others were surprisingly organized—for example, amorphous aggregates in the petal cell vacuoles that had been dismissed in previous electron microscopy studies as artifacts or uninteresting. The 3D view of these structures revealed a regular pattern appearing in almost every petal mesophyll cell used in our study. When molecules are organized like this, it suggests function and poses new questions."
Electron microscopes can produce images at a much higher magnification and resolution than optical light microscopes because electrons have shorter wavelengths than visible light. The ion beam is able to penetrate the tissue sample and slice off thin sections at a precision much greater than any form of mechanical milling. Combining these two technologies offers unique images unattainable by any other method.
Unfortunately, all good things tend to have their drawbacks and FIB-SEM is no exception. It is a time-intensive process that requires an expensive specialized instrument. Tissue samples must undergo highly specific fixation procedures to stabilize them for imaging in the electron microscope, and non-conductive biological cells require a conductive coating, such as platinum or a gold alloy.
For the research team at MTSU, the time-consuming procedures and expensive equipment are well worth the results. "We have new specific questions to address that arose from the expanded view of the internal structures. In addition, now that we know how to use this technology, we have begun to expand the repertoire of photosynthetic organisms to, at least initially, explore their cellular architecture just to see where it takes us."
The new FIB-SEM methods developed for seed, leaf, stem, root, and petal cell types will help expand the toolset available to plant anatomists for understanding the nature of organelles, cells, and plant development. A unique view of plant cell interiors could reveal never-before-seen aspects of the architecture and distribution of organelles. This work is just one example of how technological advances in one field of science, in this case, materials science, can open new doors for researchers in other fields.
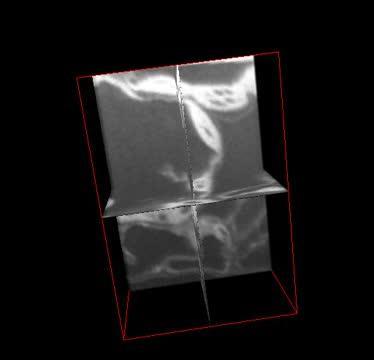
This shows 3-D ortho-rotation of leaf mesophyll cells. Micrographs were collected by milling fixed tissue accompanied by SEM imaging using FIB-SEM. The complete videos published with the article are available on the Botanical Society of America's YouTube channel (Video 1: http://youtu.be/0nnqMBJlLA0; Video 2: http://youtu.be/B3tEY8X85f4).
(Photo Credit: Image Bhawana et al. From Bhawana, Joyce L. Miller, and A. Bruce Cahoon. 3D Plant cell architecture of Arabidopsis thaliana (Brassicaceae) using focused ion beam–scanning electron microscopy. Applications in Plant Sciences 2(6): 1300090. doi:10.3732/apps.1300090)
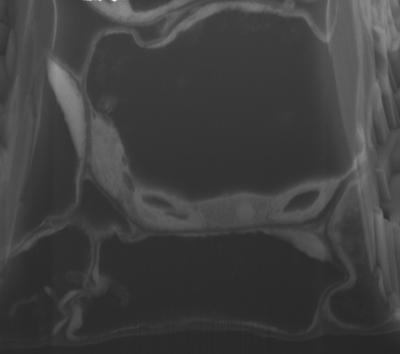
This is a high-resolution SEM micrograph from FIB-SEM manual imaging showing Arabidopsis thaliana leaf mesophyll cells with chloroplasts, nucleus, and internal membranous endoplasmic reticulum connections.
(Photo Credit: Image Bhawana et al. From Bhawana, Joyce L. Miller, and A. Bruce Cahoon. 3D Plant cell architecture of Arabidopsis thaliana (Brassicaceae) using focused ion beam–scanning electron microscopy. Applications in Plant Sciences 2(6): 1300090. doi:10.3732/apps.1300090)
Source: American Journal of Botany