AUSTIN, Texas — Thanks to a rare bacteria that grows only on rocks in the Swiss Alps, researchers at The University of Texas at Austin and the Pasteur Institute in France have been the first to identify how alcohol might affect key brain proteins.
It's a major step on the road to eventually developing drugs that could disrupt the interaction between alcohol and the brain.
"Now that we've identified this key brain protein and understand its structure, it's possible to imagine developing a drug that could block the binding site," said Adron Harris, professor of biology and director of the Waggoner Center for Alcohol and Addiction at The University of Texas at Austin.
Harris and his former postdoctoral fellow Rebecca Howard, now an assistant professor at Skidmore College, are co-authors on the paper that was recently published in Nature Communications. It describes the structure of the brain protein, called a ligand-gated ion channel, that is a key enabler of many of the primary physiological and behavioral effects of alcohol.
Harris said that for some time there has been suggestive evidence that these ion channels are important binding sites for alcohol. Researchers couldn't prove it, however, because they couldn't crystallize the brain protein well enough, and therefore couldn't use X-ray crystallography to determine the structure of the protein with and without alcohol present.
"For many of us in the alcohol field, this has been a Holy Grail, actually finding a binding site for alcohol on the brain proteins and showing it with X-ray crystallography," said Harris. "But it hasn't been possible because it is not possible to get a nice crystal."
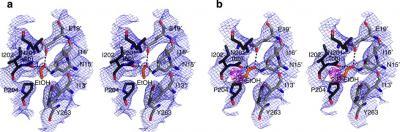
This shows: (a) Stereo view of protein atoms neighbouring one ethanol molecule (orange) in the co-crystal structure with GLIC F14′A. Dotted lines indicate putative hydrogen bonds with the backbone carbonyl of N200 in the left-hand subunit (dark grey) and with N15′ and E19′ in the right-hand subunit (light grey). (b) Protein atoms neighboring one molecule of 2-bromoethanol (orange) in the co-crystal structure with GLIC F14′A, displayed as in a. Sigma-A-averaged electron density surrounding ethanol and 2-bromoethanol are contoured at 1.0 σ (blue mesh) and bromine-anomalous map is contoured at 5.0 σ (magenta mesh).
(Photo Credit: Nature Communications)
The breakthrough came when Marc Delarue and his colleagues at the Pasteur Institute sequenced the genome of cyanobacteria Gloeobacter violaceus. They noted a protein sequence on the bacteria that is remarkably similar to the sequence of a group of ligand-gated ion channels in the human brain. They were able to crystallize this protein. Harris saw the results and immediately got in touch.
"This is something you never would have found with any sort of logical approach," he said. "You never would have guessed that this obscure bacterium would have something that looks like a brain protein in it. But the institute, because of Pasteur's fascination with bacteria, has this huge collection of obscure bacteria, and over the last few years they've been sequencing the genomes, keeping an eye out for interesting properties."
Harris and Howard asked their French colleagues to collaborate, got the cyanobacteria, changed one amino acid to make it sensitive to alcohol, and then crystallized both the original bacteria and the mutated one. They compared the two to see whether they could identify where the alcohol bound to the mutant. With further tests they confirmed that it was a meaningful site.
"Everything validated that the cavity in which the alcohol bound is important," said Harris. "It doesn't account for all the things that alcohol does, but it appears to be important for a lot of them, including some of the 'rewarding' effects and some of the negative, aversive effects."
Going forward, Harris and his lab plan to use mice to observe how changes to the key protein affect behavior when the mice consume alcohol.
They're also hoping to identify other important proteins from this family of ligand-gated ion channels. In the long term, he hopes to be involved in developing drugs that act on these proteins in ways that help people diminish or cease their drinking.
"So why do some people drink moderately and some excessively?" he said. "One reason lies in that the balance between the rewarding and the aversive effects, and that balance is different for different people, and it can change within an individual depending on their drinking patterns. Some of those effects are determined by the interactions of alcohol and these channels, so the hope is that we can alter the balance. Maybe we can diminish the reward or increase the aversive effects."
Source: University of Texas at Austin