To watch TAT at work, Dr. Roll-Mecak and her colleagues took high resolution movies of individual TAT molecules interacting with microtubules in real time. They saw that TAT surfs through the inside of microtubules and although it can find acetylation sites quickly, the process of adding the tag occurs very slowly.
In general, tagging reactions work like keys fitting into locks: the better the key fits, the faster the lock can open. Similarly, the rate of the reactions is determined by how well TAT molecules fit around tagging sites.
Dr. Roll-Mecak's team investigated this idea by using a technique called X-ray crystallography to look at how atoms on TAT molecules interact with acetylation sites on tubulin molecules. Their results suggested that TAT fit poorly around the sites.
"It looks as though TAT can easily journey through microtubules spotting acetylation sites but may only label those that are stable for longer periods of time," said Dr. Roll-Mecak.
This may help cells identify the microtubules they need to rapidly change shapes or send cargo to other places. Further studies may help researchers understand how microtubule tagging influences nerve cells in health and disease.
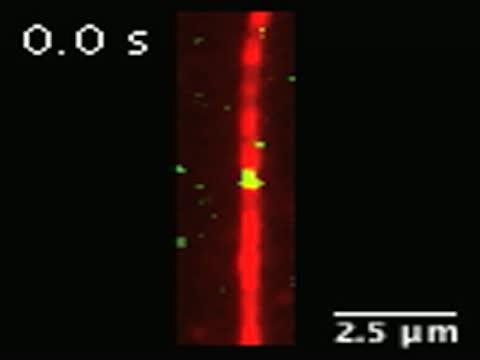
Researchers watched TAT proteins (green) journey into microtubules (red). TAT proteins are known to label the insides of the tubes. They observed that TAT can move quickly, back and forth, through microtubules while searching for sites to label.
(Photo Credit: Courtesy of the Roll-Mecak lab, NINDS)
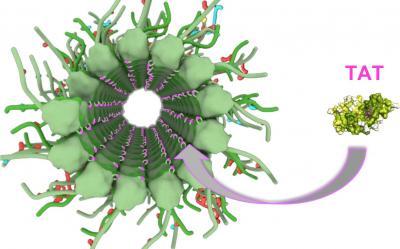
NIH scientists watched the inside of brain cell tubes, called microtubules, get tagged by a protein called TAT. Tagging is a critical process in the health and development of nerve cells.
(Photo Credit: Courtesy of the Roll-Mecak lab, NINDS, Bethesda, MD)
Source: NIH/National Institute of Neurological Disorders and Stroke