With a nod to one of nature's best surface chemists—an obscure desert beetle—polymer scientists at the National Institute of Standards and Technology (NIST) have devised a convenient way to construct test surfaces with a variable affinity for water, so that the same surface can range from superhydrophilic to superhydrophobic, and everything in between. Their technique, reported in a recent issue of the journal Langmuir,* may be used for rapid evaluation of paints and other materials that need to stick to surfaces.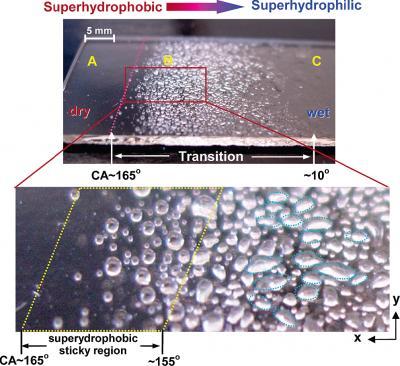 Wetability gradient: Water sprayed on a glass slide coated with a nanostructured gradient wettability film using the new NIST technique illustrates the transition from (A) superhydrophobicity to (C) superhydrophilicity. The lower image shows the magnified image of the (A) hydrophobic to (B) transition wetting region. The pink dotted line indicates the border of the superhydrophobic region, and the yellow dotted region shows a hydrophobic 'sticky' region. Credit: NIST
Wetability gradient: Water sprayed on a glass slide coated with a nanostructured gradient wettability film using the new NIST technique illustrates the transition from (A) superhydrophobicity to (C) superhydrophilicity. The lower image shows the magnified image of the (A) hydrophobic to (B) transition wetting region. The pink dotted line indicates the border of the superhydrophobic region, and the yellow dotted region shows a hydrophobic 'sticky' region. Credit: NIST
The NIST team developed a flexible technique, based on ultraviolet light and photosensitive materials, to mimic one of nature's cleverest feats of surface chemistry. The Stenocara beetle of Africa's Namib Desert is able to thrive in a habitat so parched that not even the morning fog will condense. All the beetle has to do is raise its warty-looking wing covers into the breeze. Because the bumps are hydrophilic, or water-attracting, while the rest of the surface is hydrophobic, or water-repelling, the few water molecules that do strike the wing covers tend to get pushed uphill and collect on the bumps—where they eventually condense into artificial dewdrops that roll into the insect's mouth. The insect's trick is to use both surface structure and chemistry to create a surface that shifts rapidly from hydrophobic to hydrophilic.
The NIST researchers begin by coating the surface with a matrix of silica granules about 11 nanometers across. As with the beetle, whose wing covers are coated with organic particles about a thousand times larger, the spacing of the matrix provides a first, purely physical level of control over wettability: a water droplet placed atop the granules can sag only just so far into the gaps before it is stopped by surface tension.
The researchers then add a second level of control by coating the granules with a compound that changes their water affinity, in much the same way that a waxy substance makes some of the beetle's microparticles hydrophobic. This step in itself is not unique; other research groups have added such compounds to granular surfaces using electrochemical techniques. The NIST group's innovation is to use an optical technique that is much easier to modulate, and that can be carried out in air. They simply coat the granules with a photosensitive material, and expose it to ultraviolet light: the longer and more intense the exposure in a given area, the more hydrophilic that area becomes.
The new technique's most immediate application is for testing paints, adhesives and other coatings: instead of daubing the compounds on dozens of surfaces one by one, researchers can now spread them over a single surface that tests the entire range of wettability within the space of a few centimeters. Other applications also are possible, ranging from water collection in dry regions to open-air microchannel devices. Indeed, the same technique can be used to create surfaces that vary in their affinity for alcohol and many other small molecule liquids.
Source: J.T. Han, S. Kim and A. Karim. UVO-tunable superhydrophobic to superhydrophilic wetting transition on biomimetic nanostructured surfaces. Langmuir 2007, 23, 2608-2614.