The researchers managed to overcome other disadvantages of traditional photocatalytic systems. "People have typically used catalysts made from platinum and other expensive metals," Holland said. "It would be much more sustainable if we used metals that were more easily found on the Earth, more affordable, and lower in toxicity. That would include metals, such as nickel."
Holland said their work is still in the "basic research stage," making it impossible to provide cost comparisons with other energy production systems. But he points out that nickel currently sells for about $8 per pound, while the cost of platinum is $24,000 per pound.
While all three researchers say the commercial implementation of their work is years off, Holland points out that an efficient, low-cost system would have uses beyond energy. "Any industry that requires large amounts of hydrogen would benefit, including pharmaceuticals and fertilizers," said Holland.
The process developed by Holland, Eisenberg, and Krauss is similar to other photocatalytic systems; they needed a chromophore (the light-absorbing material), a catalyst to combine protons and electrons, and a solution, which in this case is water. Krauss, an expert in nanocrystals, provided cadmium selenide (CdSe) quantum dots (nanocrystals) as the chromophore. Holland, whose expertise lies in catalysis and nickel research, supplied a nickel catalyst (nickel nitrate). The nanocrystals were capped with DHLA (dihydrolipoic acid) to make them soluble, and ascorbic acid was added to the water as an electron donor.
Photons from a light source excite electrons in the nanocrystals and transfer them to the nickel catalyst. When two electrons are available, they combine on the catalyst with protons from water, to form a hydrogen molecule (H2).
This system was so robust that it kept producing hydrogen until the source of electrons was removed after two weeks. "Presumably, it could continue even longer, but we ran out of patience!" said Holland.
One of the next steps will be to look at the nature of the nanocrystal. "Some nanocrystals are like M&Ms – they have a core with a shell around it," said Eisenberg. "Ours is just like the core. So we need to consider if they would they work better if they were enclosed in shells."

Nanocrystals and nickel catalyst substantially improve light-based hydrogen production.
(Photo Credit: Matthew Mann/University of Rochester)
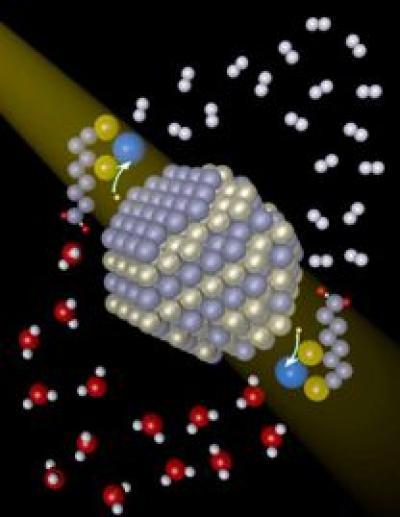
Cadmium selenide nanocrystals absorb light and transfer electrons to a nickel catalyst (blue), which subsequently generates hydrogen (white).
(Photo Credit: Ted Pawlicki/University of Rochester)
Source: University of Rochester