Bottled sun
This high efficiency provides stiff competition for other techniques used to convert solar energy. But this method has several advantages over others:
"Both the perovskite used in the cells and the nickel and iron catalysts making up the electrodes require resources that are abundant on Earth and that are also cheap," explained Jingshan Luo. "However, our electrodes work just as well as the expensive platinum-based models customarily used."
On the other hand, the conversion of solar energy into hydrogen makes its storage possible, which addresses one of the biggest disadvantages faced by renewable electricity—the requirement to use it at the time it is produced.
"Once you have hydrogen, you store it in a bottle and you can do with it whatever you want to, whenever you want it," said Michael Grätzel. Such a gas can indeed be burned – in a boiler or engine – releasing only water vapor. It can also pass into a fuel cell to generate electricity on demand. And the 12.3% conversion efficiency achieved at EPFL "will soon get even higher," promised Grätzel.
Science published on Sept. 25, 2014 the latest developments in Michael Grätzel's laboratory at EPFL in the field of hydrogen production from water. By combining a pair of perovskite solar cells and low price electrodes without using rare metals, scientists have obtained a 12.3% conversion efficiency from solar energy to hydrogen, a record with earth-abundant materials. Jingshan Luo, post-doctoral researcher, explains how.
(Photo Credit: EPFL)
More powerful cells
These high efficiency values are based on a characteristic of perovskite cells: their ability to generate an open circuit voltage greater than 1 V (silicon cells stop at 0.7 V, for comparison).
"A voltage of 1.7 V or more is required for water electrolysis to occur and to obtain exploitable gases," explained Jingshan Luo. To get these numbers, three or more silicon cells are needed, whereas just two perovskite cells are enough. As a result, there is more efficiency with respect to the surface of the light absorbers required. "This is the first time we have been able to get hydrogen through electrolysis with only two cells!" Luo adds.
The profusion of tiny bubbles escaping from the electrodes as soon as the solar cells are exposed to light say it better than words ever could: the combination of sun and water paves a promising and effervescent way for developing the energy of the future.
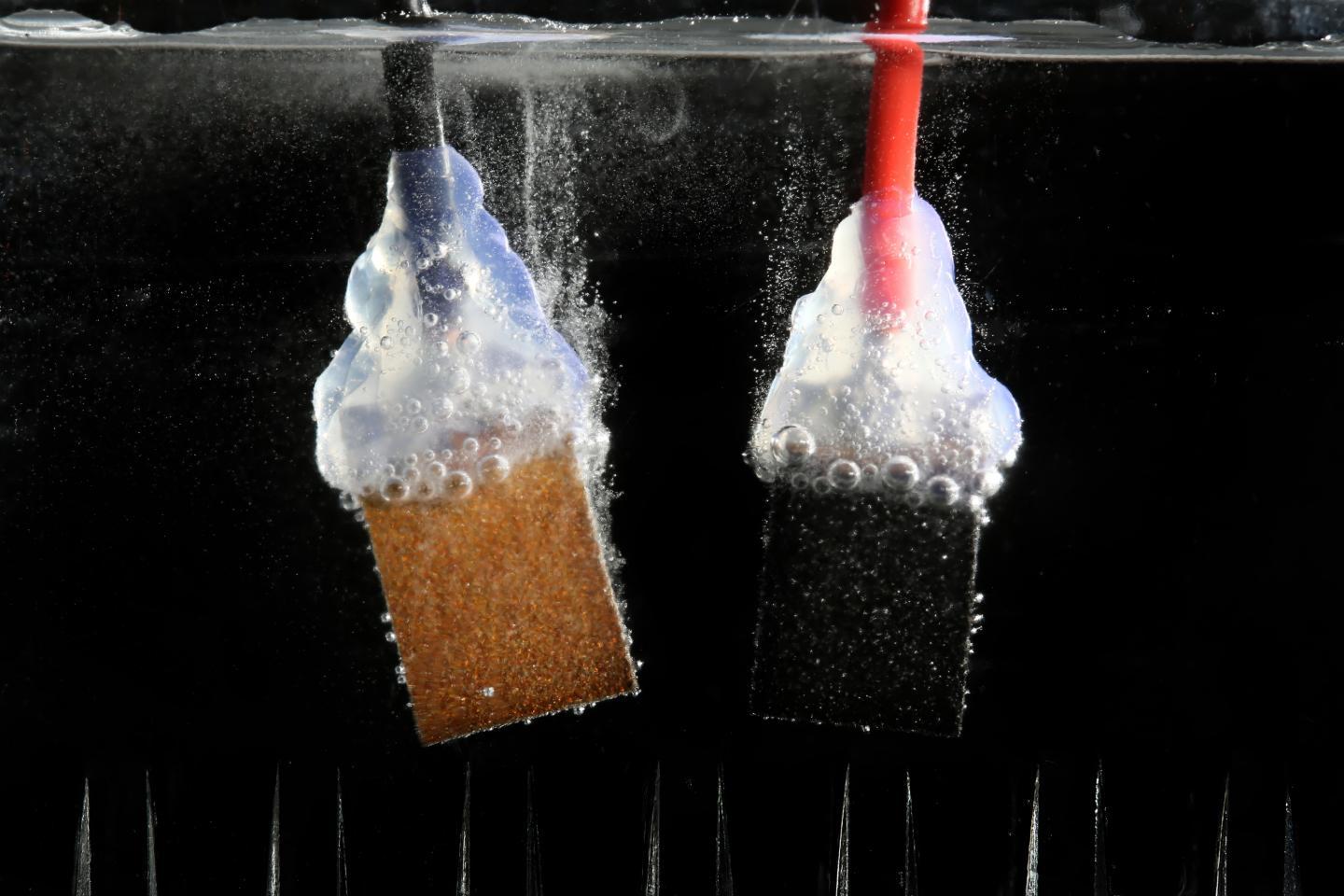
When an electrical current is provided, water splits into oxygen and hydrogen.
(Photo Credit: EPFL / LPI / Alain Herzog)

When an electrical current is applied, water splits into hydrogen and oxygen.
(Photo Credit: EPFL / LPI / Alain Herzog)