LA JOLLA, Calif., December 26, 2013 – Sanford-Burnham Medical Research Institute scientists have discovered a new molecule that forms when certain white blood cells—macrophages—are stimulated in response to pathogens. The molecule, termed "THRIL," helps regulate the immune response and shows an association with Kawasaki disease. The findings suggest that THRIL may contribute to other inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease.
The study, published online in Proceedings of the National Academy of Sciences, measured large intergenic noncoding RNA (lincRNA) produced when the immune system is activated. One lincRNA was found to bind heterogenous nuclear ribonucleoprotein L (hnRNPL), creating a new molecule that regulates genetic control of TNF-alpha—a potent cytokine that promotes inflammation. The authors named the molecule THRIL, after TNF-alpha and hnRNPL-related immunoregulatory lincRNA.
Noncoding RNAs as key regulators of immune response
Large noncoding RNA corresponds to the parts of the genome that do not code for protein.
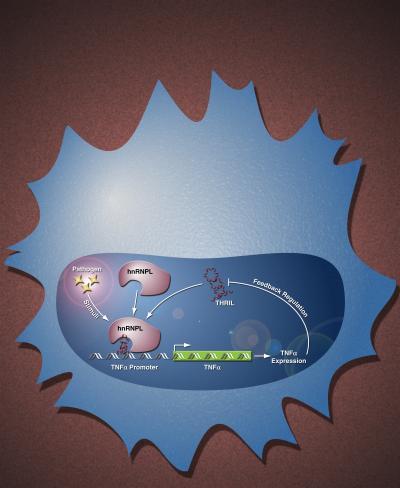
This is the mechanism of action of 'THRIL' -- a newly discoverd TNF-alpha regulator.
(Photo Credit: RANA)
"For some time we have known that noncoding regions of RNA play important roles in regulating the immune response to microbial pathogens," said Tariq Rana, Ph.D., senior author of the study and professor in the Sanford Children's Health Research Center and director of the RNA Biology Program at Sanford-Burnham. "When we realized that THRIL functioned to control the TNF-alpha gene, we wanted to see if it mirrors the progression in inflammatory diseases."
Collaborating with Jane Burns, M.D., professor of pediatrics at Rady Children's Hospital and UC San Diego, Rana's team measured THRIL levels in Kawasaki disease samples at different stages of the disease, and found that levels were at their lowest during the acute stage of the disease—when TNF-alpha levels are at their highest.
The findings suggest that THRIL could be a novel biomarker for immune activation and a potential target for inflammatory diseases.