The results obtained by Rune T. Kidmose and Nick S. Laursen are remarkable. "It's extremely rare that you can trap a proteolytic (protein-degrading) enzyme in the middle of the process of cleaving an intact protein," says Associate Professor Gregers Rom Andersen, the students' supervisor. "We now know in detail which parts of MASP-2 recognise the substrate C4. Another fascinating aspect of their results is that we also know the structure of both C4 and MASP-2 alone, so we can see how both the enzyme and the substrate change their three-dimensional structure when C4 is recognised by MASP-2. We can also see how these changes directly contribute to facilitating the cleavage of C4," he concludes.
For Professor Thiel, the new results represent a completely new way to visualise how MASP-2 – whose function he discovered in 1997 – carries out its function. "It's also a great personal pleasure to be involved right from the discovery of a new protein to the point where you obtain knowledge at the atomic level on how this protein functions as an enzyme," he says.
In many situations, an undesirable activation of the complement system takes place, which can damage our own tissues. Several pharmaceutical companies are currently working on developing drugs that can attenuate such damage. "The very detailed understanding that we now have concerning parts of the complement system will undoubtedly lead to more intelligent ways of developing new drugs," says Professor Thiel. "Of course, we won't stop our studies here, as we'll continue the very detailed studies of other proteins and molecular mechanisms within the immune system," he continues.
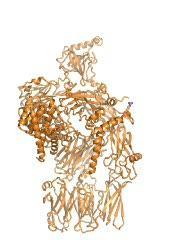
The animation shows how the MASP-2 (blue) attaches itself to the complement protein C4 (orange), and the structural rearrangements that occur in the C4 due to this. Note that although the MASP-2 recognizes only a relatively small part of the C4, rearrangements take place in large areas of the C4 molecule.
(Photo Credit: Rune T. Kidmose)
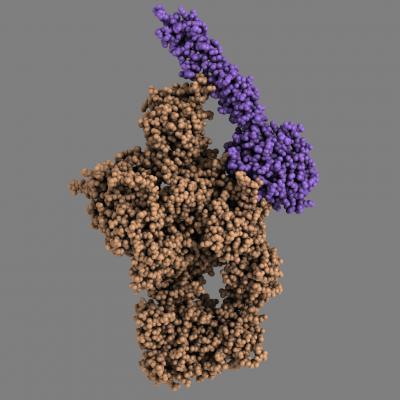
The model shows how the MASP-2 attaches itself to the C4, which allows the MASP-2 to cleave a small portion of the C4. This makes the structure of C4 change, which enables the C4 to bind to the surface of pathogenic microorganisms, for example, or our own dying cells
(Photo Credit: Rune T. Kidmose)
Source: Aarhus University