This work indicates how Cdc42 activates bipolar growth only once a minimal cell length has been achieved. At that point, Cdc42 begins to oscillate back and forth through the cell, as the two tips compete for it. Using fluorescent markers to tag each of the many proteins involved, researchers observed the Cdc42 protein oscillate from side to side within a cell, switching sides about every five minutes. The fluctuations provide an adaptable mechanism for cells to control their size and structure in the fast-changing environment within.
The study, Oscillatory Dynamics of Cdc42 GTPase In The Control of Polarized Growth, appears today in the journal Science.
The findings demonstrate just part of the complex process of cell growth and differentiation, but mark how advanced the science of biophysics has become. Only recently has the clear imaging and monitoring of protein activity become possible at the minute sizes and shortened time scales of individual cell maturation.
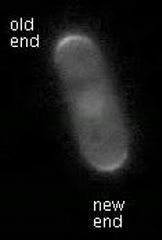
This video shows active Cdc42 oscillating through yeast cells.
(Photo Credit: University of Miami Miller School of Medicine)
"Up until now, no one has ever seen the way this protein oscillates back and forth throughout the cell," said Tyler Drake, a Lehigh graduate student and co-author of the paper. "Looking at a simple system like yeast may allow us to understand the principles behind growth in other cells."
The Lehigh team developed the mathematical model of this phenomenon by analyzing cell data collected by Maitreyi Das and Fulvia Verde at the University of Miami. Drake and Vavylonis used a Lehigh Class of 1968 Junior Faculty Fellowship and a Sigma Xi grant to visit the University, where they began to test their mathematical theory. According to the model, changes in abundance or activity of Cdc42, or of its regulators, can shift the system to more asymmetric or symmetric states. The model's conclusions were supported by biological observations of the Miami team, who genetically manipulated regulators of the protein and realized they could change cell shape and growth symmetry by adjusting Cdc42.
Vavylonis's research has for years explored the way the cellular cytoskeleton organizes and functions. In collaboration with biologists and computer scientists, his team uses physics to study, analyze, and model the physical properties of these adaptive biological materials.
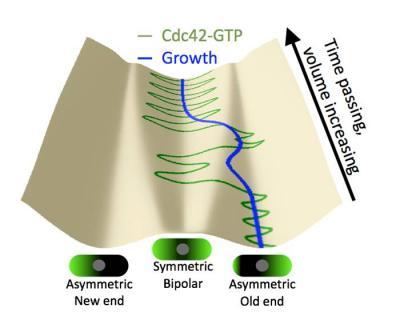
An illustration of the Lehigh mathematical model shows the evolution of Cdc42 distribution during cell growth, as cells transition toward a symmetric, or growth state.
(Photo Credit: Lehigh University)
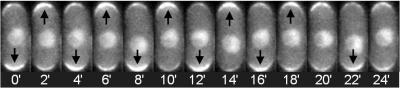
Active Cdc42 oscillates through yeast cells. The tips are sites of cell growth and the presence of active Cdc42 at the tips activates processes that contribute to cell growth.
(Photo Credit: Lehigh University)
Source: Lehigh University