In previous studies, gene circuits with predefined behaviors have been successfully built and modeled, but mostly on a case-by-case basis. In this study published in Nature Communications, researchers from the EMBL/CRG Systems Biology Research Unit at the CRG, went beyond individual networks and explored both computational and synthetic mechanisms for a complete set of 3-node stripe-forming networks in Escherichia coli. The approach combined experimental synthetic biology led by Mark Isalan, now Reader in Gene Network Engineering at the Department of Life Sciences of Imperial College London with computational modelling led by James Sharpe, ICREA Research Professor and head of the Multicellular Systems Biology lab at the CRG.
"We have performed a very innovative and ambitious study: we applied a three-step approach for the effective exploration and creation of successful synthetic gene circuits. We created a theoretical framework to study the GRNs exhaustively" - 100,000 versions of over 2800 networks were simulated on the computer. We then successfully developed a synthetic network engineering system and, finally, we confirmed all the new experimental data by fitting it to a single mathematical model" explains the corresponding author James Sharpe.
Researchers at the CRG try to understand how networks of genes work together to create specific patterns like stripes.
They have gone beyond studying individual networks and have created computational and synthetic mechanisms for a whole "design space" of networks in the bacteria Escherichia coli.
The system proves to be more efficient and powerful than building networks one-by-one, and its results have been published in the journal Nature Communications.
(Photo Credit: CRG)
First, Andreea Munteanu, co-author of the study, performed a theoretical screen for finding all design classes that produce the desired behaviour (stripe formation in a morphogen gradient). During this step she discovered four fundamentally-different mechanisms for forming a stripe. Next, Yolanda Schaerli, first author of the study, successfully demonstrated that the four networks are functional by building them in the bacteria E. coli using the tools of synthetic biology. The third step was to verify the distinct mechanisms by fitting all the experimental data to a mathematical model.
The success of this procedure allowed the researchers to go one step further to find a deeper design principle of stripe formation. They identified a simpler 2-node network – where the stripe gene is directly controlled by both activation and repression from the morphogen sensor gene– that replicates the stripe-forming ability in its simplest form. They were successful in building this archetype of stripe forming networks and ultimately discovered that it can even display an "anti-stripe" phenotype (fig. 2, bacterial lawns).
"Combining exhaustive computational modeling with synthetic biology is more efficient and powerful than building networks one-by-one" says the corresponding author Mark Isalan. "Our approach provides a new and efficient recipe for synthetic biology - a new scientific discipline which aims to engineer all kinds of useful biological systems"
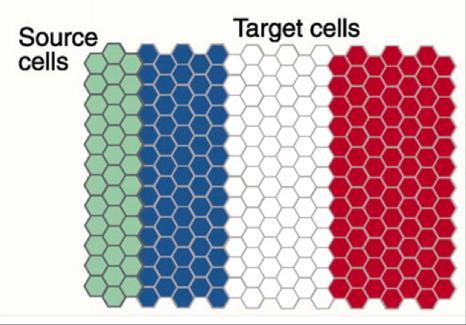
The French Flag model of stripe formation: source cells secrete a signal. Receiver cells "read" the signal concentration and make red, white or blue stripes by expressing genes.
(Photo Credit: CRG)
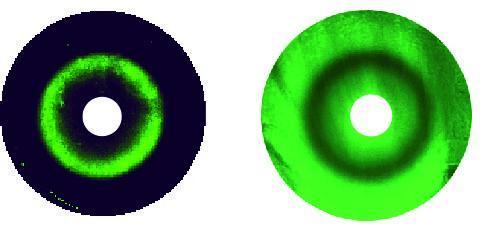
Bacterial lawns display green fluorescent ring ("circular stripe," left) as a function of a chemical gradients from central paper disks (white) or an anti-ring ("circular anti-stripe," right).
(Photo Credit: CRG)
Source: Center for Genomic Regulation