Evans' team injected doses of FGF1 into obese mice with diabetes to assess the protein's potential impact on metabolism. Researchers were stunned by what happened: they found that with a single dose, blood sugar levels quickly dropped to normal levels in all the diabetic mice.
"Many previous studies that injected FGF1 showed no effect on healthy mice," says Michael Downes, a senior staff scientist and co-corresponding author of the new work. "However, when we injected it into a diabetic mouse, we saw a dramatic improvement in glucose."
The researchers found that the FGF1 treatment had a number of advantages over the diabetes drug Actos, which is associated with side effects ranging from unwanted weight gain to dangerous heart and liver problems. Importantly, FGF1—even at high doses—did not trigger these side effects or cause glucose levels to drop to dangerously low levels, a risk factor associated with many glucose-lowering agents. Instead, the injections restored the body's own ability to naturally regulate insulin and blood sugar levels, keeping glucose amounts within a safe range—effectively reversing the core symptoms of diabetes.
"With FGF1, we really haven't seen hypoglycemia or other common side effects," says Salk postdoctoral research fellow Jae Myoung Suh, a member of Evans' lab and first author of the new paper. "It may be that FGF1 leads to a more 'normal' type of response compared to other drugs because it metabolizes quickly in the body and targets certain cell types."
The mechanism of FGF1 still isn't fully understood—nor is the mechanism of insulin resistance—but Evans' group discovered that the protein's ability to stimulate growth is independent of its effect on glucose, bringing the protein a step closer to therapeutic use.

Salk scientists explain the implications of their latest finding and how the treatment reverses symptoms of type 2 diabetes in mice without side effects.
(Photo Credit: Salk Institute for Biological Studies)
"There are many questions that emerge from this work and the avenues for investigating FGF1 in diabetes and metabolism are now wide open," Evans says. Pinning down the signaling pathways and receptors that FGF1 interacts with is one of the first questions he'd like to address. He's also planning human trials of FGF1 with collaborators, but it will take time to fine-tune the protein into a therapeutic drug.
"We want to move this to people by developing a new generation of FGF1 variants that solely affect glucose and not cell growth," he says. "If we can find the perfect variation, I think we will have on our hands a very new, very effective tool for glucose control."

These are the members of the Gene Expression Laboratory, from the left: Jae Myoung Suh, Annette Atkins, Michael Downes, Maryam Ahmadian, Ronald Evans and Ruth Yu.
(Photo Credit: Courtesy of the Salk Institute for Biological Studies)
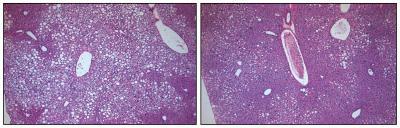
In the liver tissue of obese animals with type 2 diabetes, unhealthy, fat-filled cells are prolific (small white cells, panel A). After chronic treatment through FGF1 injections, the liver cells successfully lose fat and absorb sugar from the bloodstream (small purple cells, panel B) and more closely resemble cells of normal, non-diabetic animals.
(Photo Credit: Salk Institute for Biological Studies)
Source: Salk Institute