"We describe how tropomodulin interacts with the slow-growing end of actin filaments," says coauthor Yadaiah Madasu, PhD, a postdoctoral fellow in the Dominquez lab. "From a clinical point of view, we know that mutations in tropomodulin can trigger an accumulation of irregular actin filament bundles, which contribute to nemaline myopathy or other skeletal muscle disorders typified by delayed motor development and muscle weakness."
"The lack of structural information for the minus end of the actin filament severely limits our understanding of how tropomodulin caps actin," says Dominguez. The team described atomic crystal structures of tropomodulin complexes with actin. The structures and biochemical analysis of engineered tropomodulin variants show how one tropomodulin molecule winds around the minus end of an actin filament, making highly specific interactions with three actin subunits and two tropomyosin molecules (another protein characteristic of muscle sarcomeres) on each end of the actin filament. The detailed picture emerging from this study will help shed light on how mutations in tropomodulin, actin, and tropomysin can cause heart disorders.
The team is now studying another muscle protein called leiomodin. It was discovered more recently and resembles tropomodulin, but appears to have a completely different function, by participating in the development and repair of muscle sarcomeres.
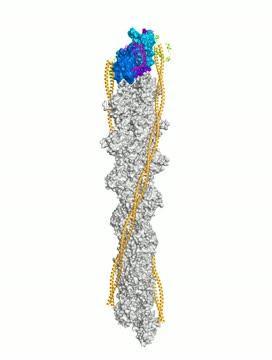
This is a representation of the atomic structure of tropomodulin at the minus end of the actin filament in muscle sarcomeres. Tropomodulin interacts with the first three actin subunits of the filament. The regions of tropomodulin that interact with actin are colored magenta, and the three actin subunits involved in this interaction are represented by two shades of blue, and purple for the interacting surface of the third subunit. Other subunits of the filament are colored gray. The actin filament also has two tropomyosin coiled coils bound symmetrically on each side (orange). At the minus end, tropomodulin also interacts with the tropomyosin coiled coils, and the region responsible for this interaction is colored green.
(Photo Credit: Yadaiah Madasu, Ph.D., Perelman School of Medicine, University of Pennsylvania, Science)
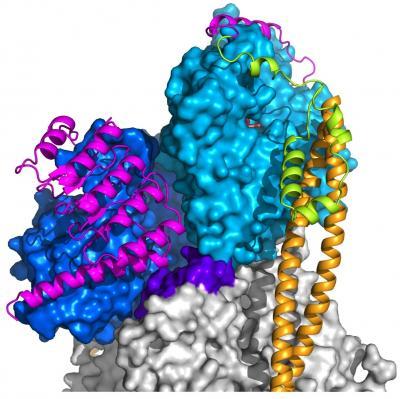
This is a representation of the atomic structure of tropomodulin at the minus end of the actin filament in muscle sarcomeres. Tropomodulin interacts with the first three actin subunits of the filament. The regions of tropomodulin that interact with actin are colored magenta, and the three actin subunits involved in this interaction are represented by two shades of blue, and purple for the interacting surface of the third subunit. Other subunits of the filament are colored gray. The actin filament also has two tropomyosin coiled coils bound symmetrically on each side (orange). At the minus end, tropomodulin also interacts with the tropomyosin coiled coils, and the region responsible for this interaction is colored green.
(Photo Credit: Yadaiah Madasu, Ph.D., Perelman School of Medicine at the University of Pennsylvania, Science)