Researchers at ITbM, Nagoya University developed a new nickel catalyst with a "Kabuto-like" structure that was found to catalyze the cross-coupling reaction between carbonyl compounds and readily available phenol derivatives, to form alpha-arylketones, which are found in many biologically active compounds (Kabuto = a helmet worn by Japanese samurai).
Nagoya, Japan – Professors Kenichiro Itami and Junichiro Yamaguchi of the Institute of Transformative Bio-Molecules (WPI-ITbM) and graduate students Ryosuke Takise and Kei Muto of Nagoya University have succeeded in developing a novel nickel catalyst to catalyze the cross-coupling reaction between carbonyl compounds and phenol derivatives. Phenol derivatives are known to be readily available starting materials, and this reaction has enabled the facile generation of alpha-arylketone compounds (aryl = aromatic ring; ketone = a carbonyl (C=O functionality with a carbon oxygen double bond) bonded to two other carbon atoms), which constitute the framework of many pharmaceuticals and organic materials. The study published online on May 20, 2014 in Angewandte Chemie International Edition, is expected to become an important tool in synthesizing biologically active molecules and a range of versatile organic materials containing the alpha-arylketone framework.
Cross-coupling reactions catalyzed by transition metals enables direct formation of carbon-carbon bonds, which is widely utilized in the formation of a vast number of organic frameworks. This strategy that won the 2010 Nobel Prize in Chemistry extends to alpha-arylation reactions, which allows incorporation of aromatic rings into carbonyl compounds through carbon-carbon bond formation, mainly using palladium as a catalyst. Many organic molecules such as amino acids contain carbonyl groups. Thus, alpha-arylation, which links carbonyl compounds to aromatic rings present in an array of organic molecules, has been used as one of the main reactions to create the organic backbone of many pharmaceuticals and organic materials. Professors Itami and Yamaguchi along with co-workers have been working to develop a new economical catalyst to conduct alpha-arylation reactions using readily available phenol derivatives as starting materials. This reaction system is envisaged to lead to the facile production of alpha-arylketones on an industrial scale.
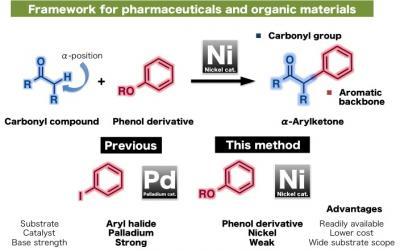
This shows aryl carbonyls as frameworks for pharmaceuticals and organic materials.
(Photo Credit: ITbM, Nagoya University)
Conventional methods for alpha-arylation have used palladium as a metal catalyst. "Since 2009, we have been looking at the potential of nickel as a cross-coupling catalyst, which is a more economical catalyst relative to palladium", says Professor Yamaguchi, one of the leaders of this research, "through our endeavors to develop improved conditions, we have found that phenol derivatives can act as readily available aryl coupling partners", he describes. Aryl substrates have mainly utilized aryl halides, which are halogenated compounds resulting in toxic wastes. Phenol derivatives on the other hand are usually less toxic and are more readily available compared to aryl halides.
Itami and his group have been working on the development of new generation cross-coupling methods using nickel as a catalyst. They have already reported various cross-coupling reactions including those between aromatic compounds and phenol derivatives, C-H coupling reactions using esters along with reactions linking aromatic rings and alkenes. These pioneering studies have been accomplished by enhancing the reactivity of nickel through tuning of various ligands. One of the effective ligands discovered in 2011 is the dcype (1,2-bisdicyclohexylphosphinoethane) ligand, which is used with nickel to activate phenol derivatives. However, the nickel(dcype) catalyst was insufficient to initiate the cross-coupling between carbonyl compounds and phenol derivatives. "We have screened various ligands and found that dcypt (1,2-bisdicyclohexylphosphinothiophene), which bears a thiophene (a 5-membered heterocycle containing sulfur) unit, acts as an effective air-stable ligand to improve the reactivity of nickel and bring about this reaction in high yield. Moreover, we were able to use a weak base to carry out this reaction, which improves the substrate scope of this reaction", explains graduate students Takise and Muto who carried out the experiments.
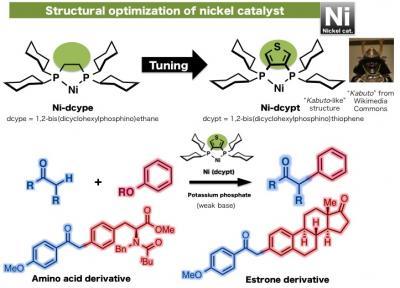
This shows structural optimization of nickel catalyst and its reaction.
(Photo Credit: ITbM, Nagoya University)
Development of this new nickel catalyst has enabled the creation of a variety of alpha-arylketone compounds, including an estrone derivative and an amino acid tyrosine derivative, both bearing the phenol moiety. Professor Itami elaborates, "Many natural products and bioactive compounds contain the alpha-arylketone framework. Our relatively low-cost nickel-dcypt catalyst has exhibited high activity to couple readily available phenol derivatives in high yield. This system has the potential to be applicable in the synthesis of molecules that can control biological systems of plants and animals, which is one of the main research themes running in our new institute (ITbM)."
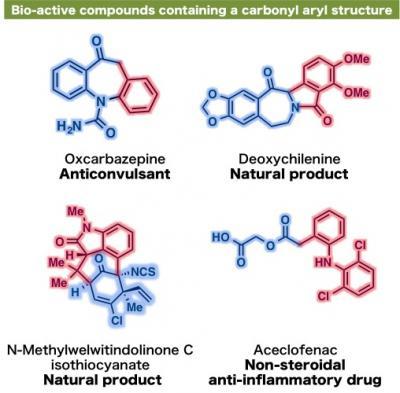
This shows examples of bio-active compounds containing a carbonyl aryl structure.
(Photo Credit: ITbM, Nagoya University)
Source: Institute of Transformative Bio-Molecules (ITbM), Nagoya University