MANHATTAN, Kan. -- A Kansas State University-led study has discovered the role of a protein in bacteria that cause a wide variety of diseases, including typhoid fever, plague, meningitis and dysentery. The results may lead to new and improved antibiotics for humans and animals.
Phillip E. Klebba, professor and head of the department of biochemistry and molecular biophysics, made the findings with two colleagues in the department: Lorne D. Jordan, doctoral candidate, Manhattan, and Salete M. Newton, research professor. The collaboration included other biophysicists at the University of Oklahoma and Purdue University. Their study, "Energy-dependent motion of TonB in the Gram-negative bacterial inner membrane," appears in the journal Proceedings of the National Academy of Sciences USA, or PNAS.
The research focuses on the central role of iron in biochemistry. Both animals and bacteria require iron for biological processes like energy generation and DNA, Klebba said. The iron acquisition systems of bacteria, however, contribute to infectious diseases.
"Iron is the object of a microbiological war in the human body," Klebba said. "Host proteins defend cells and tissues by sequestering the metal, and successful pathogens overcome this barrier and capture the iron. But the iron transport mechanisms of pathogenic organisms are not well understood."
The membrane protein TonB plays an indispensable role in the uptake of iron by Gram-negative bacteria -- a classification of bacteria that is more resistant to antibiotics because of a nearly impenetrable cell wall. Gram-negative bacteria can cause diseases such as Escherichia coli, Salmonella typhi, Yersinia pestis, Vibrio cholera, Brucella abortus, Neisseria meningitidis cause many diseases and clinical conditions; they all transport iron by the same mechanism that depends on the actions of TonB.
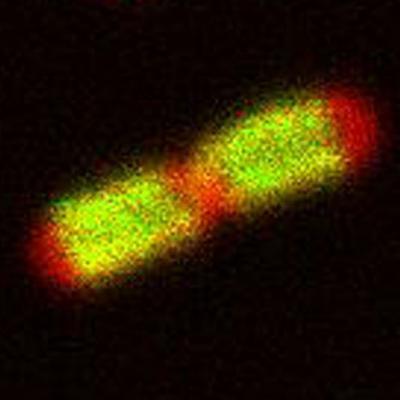
This image shows the localization of TonB (green) in E. coli cells.
(Photo Credit: Phillip E. Klebba)
Despite decades of research, the biochemical role of TonB in Gram-negative bacteria was a scientific mystery, Klebba said. He and his colleagues found that the cellular electrochemical forces put TonB in a spinning motion that provides the energy and physical mechanism to enable iron uptake into the cell.
"In this sense TonB acts like an electric motor that constantly rotates in response to the cellular energy flow," Klebba said. "TonB is one of nature's smallest and oldest electrical devices."
According to Klebba, future antibiotics may block the functions of TonB, prevent iron acquisition by Gram-negative cells, and consequently protect humans and animals from infection by such pathogen strains of bacteria.
Besides the PNAS study, Klebba recently shared the findings at the 2013 Gordon Conference on Mechanisms of Membrane Transport in South Hadley, Mass.
Source: Kansas State University