LA JOLLA, CA -- An international team led by scientists at The Scripps Research Institute (TSRI) has determined and analyzed the three-dimensional atomic structure of the human glucagon receptor. The receptor, found mainly on liver and kidney cells, helps regulate glucose levels in the bloodstream and is the target of potential therapeutic agents for type 2 diabetes.
"Our data should change the current view of how drugs are designed with this and related receptors," said TSRI Research Associate Fai Yiu Siu, PhD, who was first author of the study.
The study is reported on July 17, 2013, in an advance online edition of the journal Nature, alongside a British laboratory's structural study of another member of the same class of receptors—known as "class B" G protein-coupled receptors (GPCRs).
"Understanding how the glucagon receptor interacts with and binds to its partners will provide new information on how cells maintain sugar levels, possibly aiding the development of treatments for glucose-related disorders like type 2 diabetes," said Jean Chin, PhD, of the National Institutes of Health's National Institute of General Medical Sciences, which partially funded the research. "Because the receptor is the first in its class of membrane proteins to be structurally determined, the work may advance studies of similarly shaped, medically important but often difficult to characterize molecules."
TSRI Professor Raymond C. Stevens, PhD, who was a senior author of the study, noted, "This work involved a very fruitful international collaboration in which researchers in the United States, China and Europe worked closely together for more than two years to uncover the key differences in this subfamily of GPCRs."
Popular Drug Targets
GPCRs are the largest family of cellular receptors in humans and other animals. More than a third of all modern pharmaceuticals target these receptors, either to boost or block their activities. Determining the structural details of individual GPCRs and how they interact with binding partners has thus been a major goal of much biological research.
Over the past decade, the Stevens and Cherezov laboratories at TSRI have pioneered techniques to express, stabilize and induce these inherently flexible receptor proteins to form regular crystalline solids—from which their structures can be derived using a technique called X-ray crystallography. The crystal structures the team has "determined" so far include the groundbreaking crystallization and structure determination work of the β2 adrenergic receptor, followed by the A2A adenosine receptor (active and inactive state), S1P1 receptor, CXCR4 chemokine receptor, D3 dopamine receptor, histamine H1 receptor, kappa opioid receptor, nociception receptor, serotonin receptors 5HT1b and 5HT2b, and the smoothened receptor (a class F receptor)—all well-known and important therapeutic targets to treat a variety of human diseases. Each of these receptor systems is being followed up by careful structure-function studies including NMR in collaboration with the TSRI Wüthrich laboratory to fully understand how they work.
Other than the class F smoothened receptor, all the GPCRs whose structures have been solved to date are known as "class A" GPCRs for their common structural and protein-sequence features. More challenging for structural biologists has been the class B GPCRs, which include the glucagon receptor as well as several closely related protein molecules.
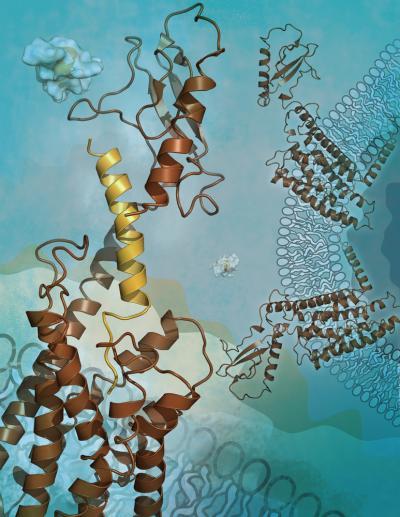
The new study shows the structure of the human glucagon receptor, which could help scientists design new drugs for type 2 diabetes.
(Photo Credit: Image courtesy of Katya Kadyshevskaya, The Scripps Research Institute.)
"These receptors are very different from the class A receptors since they have key functional domains both embedded in and outside of the cellular membrane, so the lessons we've learned from the class A have not been entirely applicable," said Vsevolod Katritch, an assistant professor of molecular biology at TSRI and a co-author on the study.
Potential Way to Control Blood Sugar
The glucagon receptor and related GLP1 and GIP receptors were high on the list of desired class B GPCR structures due to their potential as a drug targets for diabetes and their similarity to other receptors involved in endocrine and metabolic disorders. When activated by the hormone glucagon during fasting, the glucagon receptor triggers the release into the bloodstream of stored glucose from the liver and other sites. Modulating the glucagon receptor's activity thus offers a way to control blood sugar.
Other laboratories have reported finding the crystal structure of the small soluble part of the glucagon receptor, known as the extracellular domain. But the structure of the receptor's midsection, normally anchored in the host cell's membrane where the signal is transmitted, has been elusive. Although it is also involved in binding to glucagon, this "transmembrane domain" of the receptor—which resembles a jumble of seven loose springs—tends to resist the crystallization that is needed for structure determination.
After many attempts, Siu was finally able to obtain crystallography-worthy crystals of the receptor's transmembrane domain. Borrowing one key innovation from class A GPCR studies, he used a special "fusion protein" to hold the molecule together. The resulting structure, determined to a resolution of 3.4 Angstroms, turned out to have two key features that differ from those seen in class A GPCRs. One is an unusually elongated, stalk-like segment that connects the transmembrane region to the outermost, knob-like domain of the receptor. The other is an unusually large "pocket" within the transmembrane region where the N-terminal part of the glucagon peptide is expected to dock.
"If you're trying to get a drug molecule to fit snugly into that pocket, you might need a larger one than those that are normally used to target class A GPCRs," Siu said. He added that several pharmaceutical companies have been trying to develop drugs that specifically target this and related receptors. "Other than peptides, maybe the drugs they're designing need to be bigger and not conform to the usual characteristics of other drugs."
Putting Together Pieces of the Puzzle
One of the other tour de forces of the study included extensive analyses of how the receptor's glucagon-binding properties change when its individual amino acids are mutated. This work, involving the study of more than 100 separate mutations to the receptor, was performed by co-senior author Ming-Wei Wang's laboratory at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Chris de Graaf's laboratory at the Amsterdam Institute for Molecules, Medicines and Systems (AIMMS) of VU University Amsterdam, and Jesper Lau at Novo Nordisk.
Combining the new data with previous work, computational biochemists De Graaf and Katritch were able to develop a detailed model for how the full-length glucagon receptor operates: its outermost domain grabs one end of the glucagon peptide, then inserts the other end of the peptide into the large binding pocket in the transmembrane domain, locking the receptor structure in place and triggering receptor activation. "We managed to put together the pieces of this puzzle, and the mutational analysis was a key part of our model," said Stevens.
Siu, Katritch, Stevens and their colleagues now are trying to determine the glucagon receptor structure while the receptor is bound to glucagon, and at a sharper resolution.
"This glucagon receptor structure has opened the door to understanding how hormone peptides bind to this class of receptors, and it will help us solve related receptor structures," Stevens said. "Our knowledge of GPCRs is still in its infancy, and we are learning a great deal with each new structure, with different techniques and in the different functional states."
Source: Scripps Research Institute