Penn State researchers will soon provide he first demonstration of a fundamentally new method for measuring a particular quantum property of individual atoms. "This method allows us to directly and precisely measure the phase shifts that result when ultracold atoms collide, in a way that is independent of the accuracy-limiting density of the atoms," says Kurt Gibble, an associate professor of physics and principal investigator of the Penn State University research team that developed the method.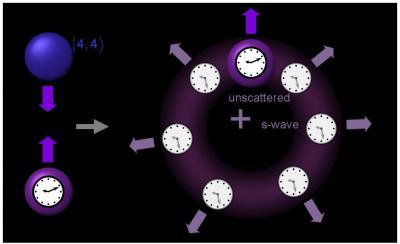 Schematic of the Experiment. We collide a clock atom with atoms in another state (labeled |4, 4>). When the clock atoms collide, they experience a discrete jump, which is the difference of two s-wave phase shifts. The s-wave phase shifts can be measured with atomic-clock accuracy and give precise information about the atom-atom interactions. Credit: Kurt Gibble, Penn State
Schematic of the Experiment. We collide a clock atom with atoms in another state (labeled |4, 4>). When the clock atoms collide, they experience a discrete jump, which is the difference of two s-wave phase shifts. The s-wave phase shifts can be measured with atomic-clock accuracy and give precise information about the atom-atom interactions. Credit: Kurt Gibble, Penn State
The researchers developed an innovative way to study atomic collisions in a cesium fountain clock -- the kind of atomic clock used to keep the world's standard of time. Atomic clocks use the quantum oscillations of ultra-cold atoms, which tick at regular intervals, to gauge the passage of time. The Penn State team was able to measure accurately the shift in the atom's quantum oscillations, or phase shifts, that it experiences during a collision with another atom.
These phase shifts, which cause jumps in the atom's ticks, limit the accuracy of the world's most accurate atomic clocks. Until this study, these shifts had been impossible to measure with high precision because earlier techniques relied on knowing the atom's density, which cannot be measured accurately. "Atomic clocks detect the entire wave-function of the atom, and this gives a frequency shift that is proportional to the density of the atoms," Gibble explains. "But, in our new technique, we detect only the part of each atom's wave function that is scattered during a collision with another atom, and these atoms see a huge frequency shift that is independent of the density of the atoms."
The method detects s-wave shifts, or jumps in time, that the atoms experience during a collision, which Gibble explains "makes some probability for the atoms to travel spherically outward, which is known as an s-wave." These s-wave phase shifts are of vital importance in many areas of research in contemporary atomic physics; for example, efforts to make use of such exotic concoctions as Bose-Einstein condensates and degenerate Fermi gasses. "The dreams for Bose-Einstein condensates include using them to make an atom laser that would be orders of magnitude more sensitive than a regular laser to enable ultra-precise navigation and measuring gravity so precisely that you perhaps could detect oil or other gravity anomalies underground," Gibble explains. "Degenerate Fermi gasses may shed light on important condensed-matter physics problems, including high-temperature superconductivity, which could have enormous technological implications including dramatically improving the efficiency and speed of electronic circuits and computers."
The research also could help scientists understand how the very early universe may have behaved differently from the way it behaves today. "Our method will give the most direct and precise measurement of ultracold interactions between atoms and will place stringent limits on a fundamental constant that some cosmologists say is likely to have had a large change over cosmic time -- the electron-proton mass ratio," Gibble says.
The experiment by Gibble's research group involves juggling two ball-shaped clouds of cesium atoms in an atomic fountain clock. Each cloud is about the diameter of a penny and contains about a billion atoms. Before the atoms in the clouds collide, they approach each other like balls on a pool table but, because quantum physics rules their behavior, they act nothing like balls on a pool table after they collide. The atoms pass through each other undeflected, without any change in their direction, as if they were unaffected by the collision. At the same time, they also "scatter" by radiating outwardly as a symmetric sphere centered at the point where they collided. This outwardly expanding sphere is known as an s-wave. "Each atom is in the quantum-mechanical superposition of being both scattered and undeflected after a collision at these extremely low temperatures," Gibble explains.
At temperatures that are a millionth of a degree above absolute zero, quantum effects dominate the atomic collisions. "In atomic fountains, the atoms are so cold that they collide at such low energies that they don't have enough energy to have even one quantum unit of angular momentum about one another," Gibble explains. This condition greatly simplifies their scattering by producing just a spherical s-wave "The only effect of a collision at low energies is a shift of the phase of the outgoing spherical s-wave, which we are able to isolate and detect with a couple of laser pulses," Gibble says.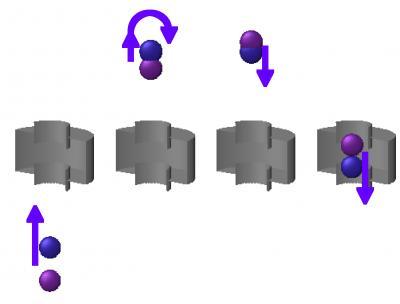 Schematic of Fountain Clock Juggling Cesium Atoms. Two clouds of cesium atoms are tossed upward with a short time delay so that they collide after passing through the microwave cavity (silver) on the way up and before they return down through the cavity. The microwaves in the cavity excite and probe the atoms, resulting in an atomic fountain clock. Credit: Kurt Gibble, Penn State
Schematic of Fountain Clock Juggling Cesium Atoms. Two clouds of cesium atoms are tossed upward with a short time delay so that they collide after passing through the microwave cavity (silver) on the way up and before they return down through the cavity. The microwaves in the cavity excite and probe the atoms, resulting in an atomic fountain clock. Credit: Kurt Gibble, Penn State
In atomic fountain clocks, clouds of atoms rise and fall like water droplets in a water fountain. But unlike water fountains, atomic clocks include a region of microwaves that the atoms pass through twice, once as they are shooting up and a second time as they are falling back down. The microwaves allow the tick rate of the atoms to be detected. "By comparing the phase of the microwaves with the phase of the s-waves of the scattered atoms detected after a collision, we can directly see the phase shifts that occur when the s-waves scatter," Gibble explains. "This new technique can be used with a wide variety of atoms, and our first results already are comparable to the best results from previous techniques. Future versions may be 100 times more accurate."
In addition to Gibble, other scientists on the Penn State research team include graduate student Russell Hart, former postdoctoral scholar Xinye Xu, now a professor at East China Normal University in Shanghai; and former graduate student Ronald Legere, now at the MIT Lincoln Laboratory in Lexington, Massachusetts.
Source: Penn State.