Researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) have found new evidence to explain how cholesteryl ester transfer protein (CETP) mediates the transfer of cholesterol from "good" high density lipoproteins (HDLs) to "bad" low density lipoproteins (LDLs). These findings point the way to the design of safer, more effective next generation CETP inhibitors that could help prevent the development of heart disease.
Gang Ren, a materials physicist and electron microscopy expert with Berkeley Lab's Molecular Foundry, a DOE nanoscience research center, led a study in which the first structural images of CETP interacting with HDLs and LDLs were recorded. The images and structural analyses support the hypothesis that cholesterol is transferred from HDLs to LDLs via a tunnel running through the center of the CETP molecule.
"Our images show that CETP is a small (53 kilodaltons) banana-shaped asymmetric molecule with a tapered N-terminal domain and a globular C-terminal domain," Ren says. "We discovered that the CETP's N-terminal penetrates HDL and its C-terminal interacts with LDL forming a ternary complex. Structure analyses lead us to hypothesize that the interaction may generate molecular forces that twist the terminals, creating pores at both ends of the CETP. These pores connect with central cavities in the CETP to form a tunnel that serves as a conduit for the movement of cholesterol from the HDL."
Ren reports the results of this study in a paper in the journal Nature Chemical Biology titled "Structure basis of transfer between lipoproteins by cholesteryl ester transfer protein." Co-authoring this paper were Lei Zhang, Feng Yan, Shengli Zhang, Dongsheng Lei, M. Arthur Charles, Giorgio Cavigiolio, Michael Oda, Ronald Krauss, Karl Weisgraber, Kerry-Anne Rye, Henry Powna and Xiayang Qiu.
Gang Ren (standing) and Lei Zhang at Berkeley Lab's Molecular Foundry were part of a team that found new evidence to explain how cholesterol is moved from HDLs to LDLs.
(Photo Credit: Photo by Roy Kaltschmidt, Berkeley Lab)
Cardiovascular or heart disease, mainly atherosclerosis, remains the leading cause of death in the United States and throughout the world. Elevated levels of LDL cholesterol and/or reduced levels of HDL cholesterol in human plasma are major risk factors for heart disease. Since CETP activity can reduce HDL-cholesterol concentrations and CETP deficiency is associated with elevated HDL-cholesterol levels, CETP inhibitors have become a highly sought-after pharmacological target for the treatment of heart disease. However, despite this intense clinical interest in CETP, little is known concerning the molecular mechanisms of CETP-mediated cholesterol transfers among lipoproteins, or even how CETP interacts with and binds to lipoproteins.
"It has been very difficult to investigate CETP mechanisms using conventional structural imaging methods because interaction with CETP can alter the size, shape and composition of lipoproteins, especially HDL," Ren says. "We were successful because we used our optimized negative-staining electron microscopy protocol that allows us to flash-fix the structure and efficiently screen more than 300 samples prepared under different conditions."
Ren and his colleagues used their optimized negative-staining electron microscopy protocol to image CETP as it interacted with spherical HDL and LDL particles. Image processing techniques yielded three-dimensional reconstructions of CETP and CETP-bound HDL. Molecular dynamic simulations were used to assess CETP molecular mobility and predict the changes that would be associated with cholesterol transfer. CETP antibodies were used to identify the CEPT interaction domains and validate the cholesterol transfer model by inhibiting CETP. This model presents inviting new targets for future CETP inhibitors.
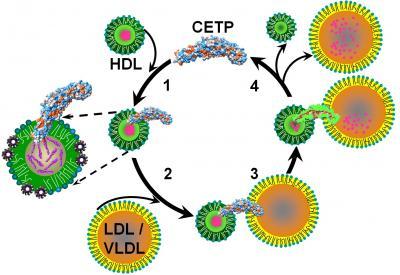
(1) CETP penetrates HDL to its cholesterol core. (2) Upon interaction with LDL/VLDL, molecular forces cause the formation of pores at either end of CETP. (3) These pores connect with CETPs central cavities to form a tunnel for the transfer of cholesterol to LDL/VLDL, which (4) reduces HDL in size.
(Photo Credit: Image by Gang Ren, Lawrence Berkeley National Laboratory)
"Our model identifies new interfaces of CETP that interact with HDL and LDL and delineates the mechanism by which the transfer of cholesterol takes place," Ren says. "This is an important step toward the rational design of next generation CETP inhibitors for treating cardiovascular disease."
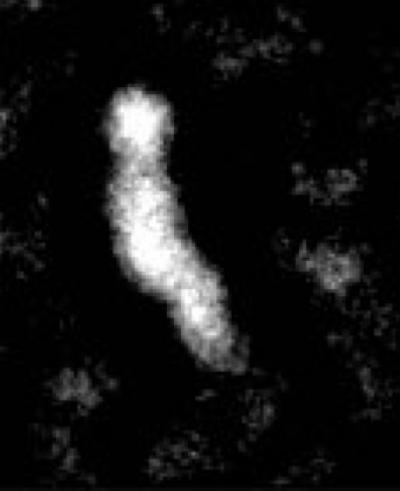
An optimized negative-staining EM of CETP shows the protein's banana shape.
(Photo Credit: (Image by Gang Ren))