Although oxygen is required to sustain life, oxygen sucks the life out of fusion by radiating away too much power from the high-temperature plasma. Accordingly, great efforts are expended to reduce the oxygen found in fusion facilities. Surprisingly, recent laboratory experiments and atomistic simulations have found that the oxygen bound by lithium at the walls of fusion devices plays a key role in improving plasma performance.
Researchers at the National Spherical Torus Experiment (NSTX) that is now being upgraded at the U.S. Department of Energy's Princeton Plasma Physics Laboratory (PPPL) have long used lithium wall conditioning as a method for improving plasma performance. These improvements include elimination of otherwise virulent edge plasma instabilities, and an improvement in the energy confinement of the plasma, both of which are correlated with a reduction of neutrals that 'recycled' at the plasma facing components. Until recently, researchers assumed that the lithium was primarily responsible for these benefits, although the precise mechanism remained unknown.
Contributing to the mystery is the fact that walls of NSTX are made of carbon in the form of graphite tiles. Lithium tends to seep into graphite, so it was unclear why any lithium would be left to capture anything that landed on the surface of the tiles. Instead, it appears that the lithium interacts with both the carbon in the tiles, and the oxygen that is naturally embedded in them, to create a new plasma-facing wall that contains all three elements. Recent studies have now shed light on how effectively this special wall surface can improve plasma performance. These studies have demonstrated the strong reaction that takes place when deuterium, the hydrogen isotope used in NSTX plasmas, comes into contact with lithium and oxygen at the plasma-facing wall of the fusion facility.
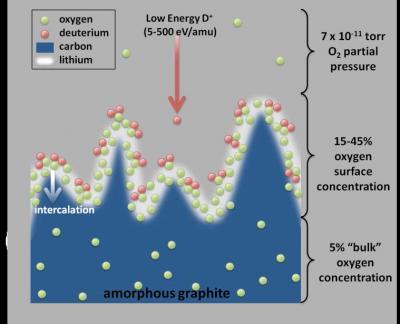
The lithium coating on graphite, which appears mountainous in this graphic done at microscopic scale, provides the perfect setting for oxygen to control the hot deuterium plasma.
(Photo Credit: C.N. Taylor and J.P. Allain)
Researchers first used a highly sensitive measurement technique called "X-ray photoelectron spectroscopy," or XPS, to detect the chemistry of the top few nanometers of the lithium-covered graphite tiles in NSTX experiments. Researchers then used computer simulations, led by P.S. Krstic, to replicate the contact between deuterium and graphite tiles impregnated with lithium and oxygen. Results showed that the lithiated and oxidized graphite captured much of the deuterium, mirroring what occurred in NSTX. (See "Deuterium Uptake in Magnetic-Fusion Devices with Lithium-Conditioned Carbon Walls" by P. S. Krstic et al. in Physical ReviewLetters 110, 105001 (2013).)
When researchers changed the simulation to eliminate the oxygen, leaving only lithium on the graphite tiles, the deuterium retention was quantitatively lower, and carbon erosion higher. As a computational exercise, the next simulation reversed the scenario to bring the deuterium into contact with a matrix of just graphite and oxygen, which would in practice be difficult to realize because of excessive oxygen contamination of the hot plasma. Surprisingly the deuterium retention was even higher than in the other two simulations.
"The combination of these simulations and experiments leads to the conclusion that lithium forms the 'glue' that allows the carbon-lithium-oxygen surface layer to very effectively retain deuterium and reduce recycling. Without the lithium, the high levels of oxygen in the surface layers needed to see this beneficial effect would likely contaminate and cool the main plasma" said physicist Chase Taylor, who led the experimental portion of the surface physics research at Purdue University with PI Prof. Jean Paul Allain who recently joined University of Illinois Urbana-Champaign. "Our results show how lithium should be prepared and maintained to yield optimum plasma performance."
Source: American Physical Society