Higher levels of cell chatter boost amyloid beta in the brain regions that Alzheimer's hits first, researchers at Washington University School of Medicine in St. Louis report. Amyloid beta is the main ingredient of the plaque lesions that are a hallmark of Alzheimer's.
These brain regions belong to a network that is more active when the brain is at rest. The discovery that cells in these regions communicate with each other more often than cells in other parts of the brain may help explain why these areas are frequently among the first to develop plaques, according to the researchers.
Working with mice genetically engineered to develop Alzheimer's type-brain changes, scientists reduced the size and number of plaques by decreasing brain cell activity in certain regions.
The results, appearing May 1 in Nature Neuroscience, are the latest to hint at a resolution to lines of evidence that have suggested busier brain cells can both contribute to and prevent Alzheimer's. According to a new theory, which brain cells are kept busy may make all the difference.
"Engaging the brain in tasks like reading, socializing or studying may be helpful because they reduce activity in susceptible regions and increase activity in regions that seem to be less vulnerable to Alzheimer's plaque deposition," says David M. Holtzman, MD, the Andrew B. and Gretchen P. Jones Professor and head of the Department of Neurology. "I suspect that sleep deprivation and increased stress, which may affect Alzheimer's risk, may also increase activity levels in these vulnerable regions."
The susceptible regions of the brain highlighted in the new study belong to the default mode network, a group of brain regions that become more active when the brain is not engaged in a cognitively demanding task. Co-author Marcus Raichle, MD, professor of neurology, of radiology and of neurobiology, was among the first to describe the default mode network.
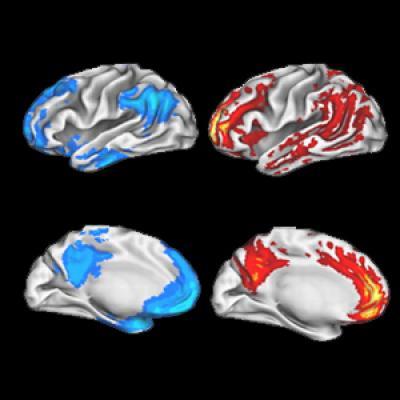
Scientists at Washington University School of Medicine in St. Louis have shown that brain cells in the default mode network, highlighted in blue on the left, communicate with each other more often than other brain areas. This may help explain why these same areas are often hit first by Alzheimer's plaques, which are highlighted in red in the brain images on the right.
(Photo Credit: Journal of Neuroscience)
In a paper published in 2005, Washington University researchers showed that regions in the default mode network are often among the first to develop Alzheimer's plaques. To understand why, Adam Bero, a graduate student in Holtzman's lab, analyzed the brain chemistry of mice. He found that the mouse brain regions analogous to those in the human default mode network had similarly high levels of early amyloid plaque deposits when compared to other areas.
Next, Bero showed in younger mice that the high-plaque regions had increased amyloid beta levels. In a third experiment, he found that the greater amyloid beta levels were caused by increased nerve cell communication in the affected regions.
To further prove the relationship between plaque formation and cell communication, scientists trimmed the whiskers on one side of a group of mice and kept them short for one month.
"Because mice are nocturnal and their eyesight is poor, whiskers are an important way for them to sense where they are in their environment," Holtzman explains. "By cutting the whiskers back on one side, we reduced neuronal activity in the region of the brain that senses whisker movement."
Loss of this input resulted in smaller and less numerous plaques on the side of the brain connected to the pruned whiskers. In a separate experiment, when researchers regularly stimulated whiskers with a cotton swab, amyloid beta levels increased.
According to Holtzman, the results demonstrate the direct connection between amyloid plaque formation and growth and changes in brain cell activity levels in various parts of the brain. He plans further investigations of the mechanisms that regulate default brain activity, their connections to phenomena such as sleep, and their potential effects on Alzheimer's disease.